SilverPlug®, the only filler for implant tunnels compliant to “MDR” EU Regulation 745/2017 “: sanctions for dentists.
A very interesting and exhaustive article by Dr. Marco Scarpelli, an Italian independent Forensic Odontologist, published by DoctorOs, definitively underlines how important and mandatory it is for dentists to use only certified medical devices for the sealing of implant screw tunnels. The EU 745/2017 Regulatory (MDR) is very clear and leaves no space for any different interpretations of the rules.
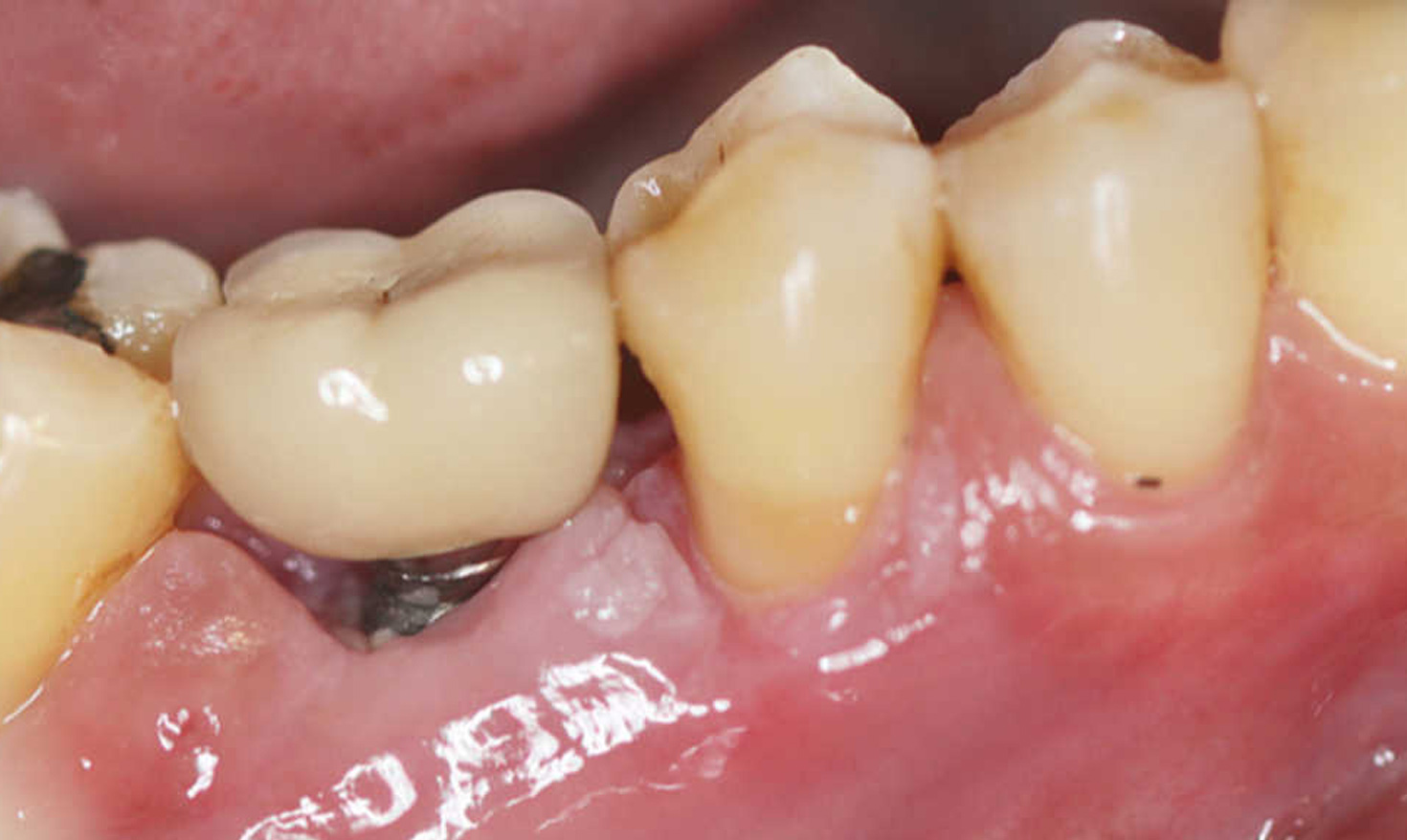
The directive clearly states that the very fact that a product is used on a patient qualifies that product as a medical device. Whoever inserts a second medical device (e.g. cotton pellets, plumber’s Teflon tape, gutta-percha or impression silicone in the screw access channel) into a main medical device (crown or abutment or Multi-Unit Abutments) is considered, for legal purposes, to be the manufacturer of a new non-certified medical device (Art. 22 para. 4) and, at the same time, the person who puts it into service (Art. 5 para. 4).
This action is, as such, punishable irrespective of whether or not the material may be harmful to the patient. Modifying a certified device, such as the implant-prosthetic system, by adding a non-certified device, such as Teflon or cotton or any other material, even certified but for a different use, therefore renders the entire device illegal.
This circumstance is the responsibility of the operator who manufactured and used the non-certified device and, in our opinion, also of the medical director (or manager) who allowed the device to be used in the first place. […] Dissuasive sanctions are applicable to all implant prostheses in which non-certified devices (Teflon, cotton, impression material, gutta-percha, etc.) have been inserted.
Non-certified medical devices, used as sealants in the case described, not only fail to comply with the rules on manufacturing, packaging and making available on the market, but also contravene the indications contained in Regulation 745/2017, Annex I Chapter 2 11: “The devices and their manufacturing processes shall be designed in such a way as to eliminate or reduce as far as possible the risks of infection for patients. The design shall be such as to: … reduce as far as possible any microbial leakage from the device and/or microbial exposure during use, and … prevent microbial contamination of the device or its contents, such as samples or fluids”.
At Silveraid, we are very proud to have foreseen the harmful implications related to non-certified implant fillers almost a decade in advance, finding a very unique and effective solution: SilverPlug® is the first and the only certified screw tunnel sealer in the word!
Enjoy the full Article, here.